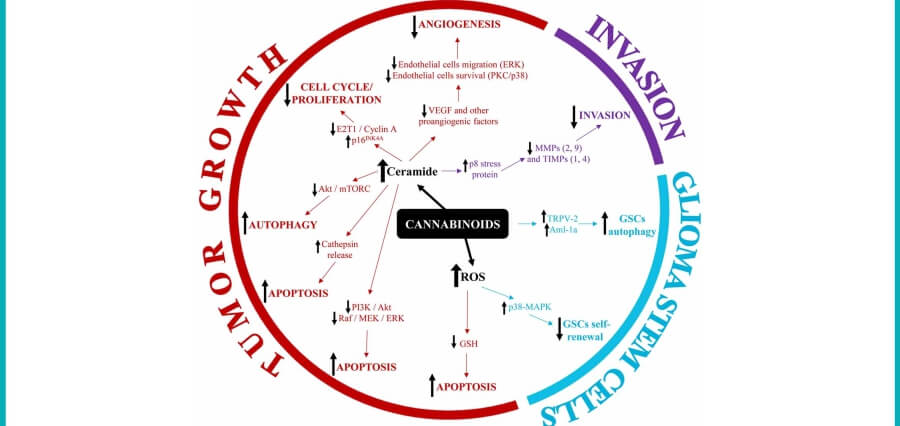
Tilray Medical, a division of Tilray Brands, is contributing to an independent clinical trial researching the efficacy of medical cannabis in treating glioblastoma, a severe form of brain cancer. The trial, set to begin on September 5, 2023, in Spain, will involve 30 patients from eight specialized neuro-oncology medical sites. Renowned scientists from the Spanish Research Group of Neuro-Oncology (GEINO) and the Complutense University of Madrid are partnering in the trial. Tilray Medical will provide pharmaceutical-grade medical cannabis (THC/CBD 1:1) for administration to patients.
The trial is Phase I, open-label, multicenter, intrapatient dose-escalation to evaluate the safety and profile of Tilray Medical’s T10:C10 (THC+CBD) extract, combined with temozolomide and radiotherapy for newly diagnosed glioblastoma patients.
Denise Faltischek, Tilray’s Chief Strategy Officer and Head of International Business emphasized the significance of the trial as a beacon of hope for patients with glioblastoma. The initiative reflects Tilray’s commitment to medical research and unlocking the therapeutic potential of medical cannabis.
The trial is unique in that it marks the culmination of a 10-year collaboration between the scientific sector and the medical cannabis community. The Medical Cannabis Bike Tour Foundation charity funds the trial. The foundation began raising funds in 2013 to support research by the Cannabinoid Signalling Group at Complutense Madrid University.
Luc Krol, founder of the Medical Cannabis Bike Tour Foundation, expressed satisfaction that the foundation can independently fund a clinical trial in the name of science. The trial is a result of civil society collaboration, an emerging pharmaceutical company, and clinical scientists.
Tilray Medical’s mission is to transform lives through safe and reliable access to medical cannabis globally. The company is a leading provider of EU-GMP-certified medical cannabis products in 20 countries.
This initiative adds to Tilray’s global support for medical trials, covering indications such as pediatric epilepsy, cancer-induced nausea and vomiting, HIV, essential tremor, breast cancer disorders, post-traumatic stress disorder, and alcohol use disorders.
The trial is a beacon of hope for patients, reflecting the continuous efforts to explore medical cannabis’s therapeutic potential in various conditions.